Under Cosmetics Supervision and Administration Regulations (CSAR) issued in 2020, companies shall apply for the registration and filing of cosmetic ingredients not listed in the Inventory of Existing Cosmetic Ingredients in China (IECIC 2021). New cosmetic ingredients refer to natural or artificial raw materials used in cosmetics for the first time in China.
Type of New Cosmetic Ingredients
New cosmetic ingredients with high safety risk(s): preservatives, colorants, anti-freckle/whitening agents, sunscreen agents, and hair dyes
New cosmetic ingredients without high safety risk(s)
Application Types
There are two types of application for new cosmetic ingredients under CSAR: registration and filing.
Registration |
|
|---|---|
Filing |
|
If the use purpose or maximum use level is changed for the approved or filed new cosmetic ingredients, new application for the registration or filing will be required.
Companies in China can apply for the registration or filing of new cosmetic ingredient directly or appoint a local agent as a technical provider to do so. Foreign companies must appoint a domestic responsible person to apply for the registration or filing of new cosmetic ingredient. The registrant or filer shall be responsible for the quality and safety of new cosmetic ingredients.
Duty of Domestic Responsible Person
Register and file the new cosmetic ingredients in the name of the registrant and filer;
Assist registrants and filers in the safety monitoring and reporting of new cosmetic raw materials;
Assist registrants and filers in the recall of cosmetic ingredients
According to the agreement between the responsible person and registrant/filer, undertaking the corresponding safety and quality responsibilities of new cosmetic ingredients placed in the Chinese market;
Cooperate with the supervision and inspection work of the supervision departments.
Application Process
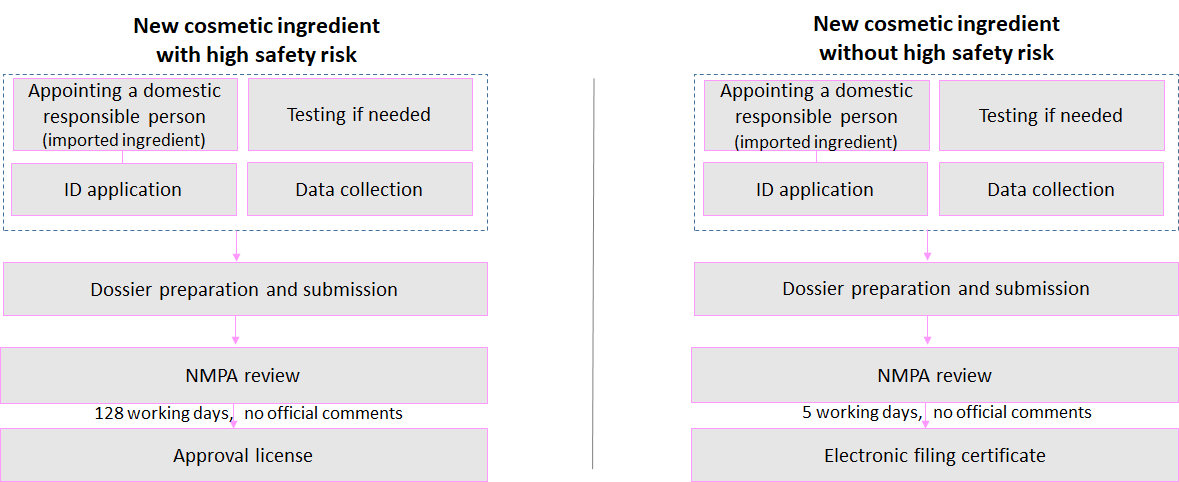
Who Shall Register?
Manufacturers of new cosmetic ingredients;
Manufacturers or brand owner of cosmetics using new cosmetic ingredients
Post-market surveillance
After registration or filing, safety monitoring system will be available to the approved or filed new cosmetic ingredients. The period of safety monitoring is 3 years from the date of completing the registration or filing of cosmetics using the approved or filed new cosmetic ingredient for the first time. During the period of safety monitoring, new cosmetic ingredient registrant or filer can use the approved or filed new cosmetic ingredient to produce cosmetics. After the 3 years of safety monitoring period, NMPA will carry out the safety evaluation for the approved or filed new cosmetic ingredients, if there is no safety issue, the approved or filed new cosmetic ingredients will be added to IECIC list. Otherwise, the registration license or filing number will be cancelled.
Our Services
CIRS China can provide all the necessary services in one package to comply with the new cosmetics regulations in China for the management of new cosmetic ingredients.
Our services include:
New Cosmetic Ingredient |
|
|---|---|
Post-notification Maintenance |
|
